Sodium is an abundant element in nature, so abundant that it is part of the “minerals” of seawater and we have daily contact with it in table salt, which is a compound called Sodium Chloride.
In agriculture, many elements are necessary for the development of plants, around 16 are essential, between macro and micronutrients. Sodium can be present in plant tissues, but it is not necessarily an essential nutrient, the plant can grow and develop in its absence. However, on some occasions, sodium can replace the functions of other elements, such as those of potassium and magnesium, especially in enzymatic activities inside the plant. The biggest problem with sodium is that it can become phytotoxic, that is, at certain relatively high concentrations, it can cause damage to plant tissue, ranging from growth retardation to plant death.
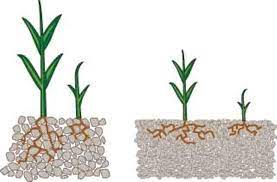
The damage occurs when sodium accumulates in the tissues, mainly on the edges of the leaves, causing burns and atrophying the tissue. This phenomenon occurs when this element is found free in the solution in the soil and is constantly absorbed by the roots of the crop and accumulated in leaves.
In order to determine the amount of Sodium in the soil, it is recommended to carry out an analysis of the saturated extract of the soil, with which the parameter called Percentage of Exchangeable Sodium (PSI, for its acronym) will be known. The PSI is a measure that represents the percentage of Na+ with respect to the other adsorbed cations.

The following table presents a list of crops with their tolerances to Sodium, represented as the measure of PSI that they can withstand:
| Sensitive | Semi-tolerant | Tolerant |
| (PSI <15 %) | (PSI 15 – 40 %) | (PSI > 40%) |
| Avocado | Carrot | Alfalfa |
| Deciduous fruit trees | Clover | Barley |
| Walnut | Fescue | Sugar beet |
| Peas | Lettuce | Cotton |
| Cotton (germinating) | Sugar cane | |
| Corn | Oats | |
| Green Bean | Onion | |
| Grapefruit | Radish | |
| Orange | Rice | |
| Peach | Rye | |
| Lentil | Italian Ryegrass | |
| Peanut | Sorghum | |
| Grasses | Spinach | |
| Tomatoes | ||
| Wheat |
In addition to the damage caused to plant tissue, mainly leaves, by the accumulation of sodium, this element causes serious problems in the soil, such as changes in structure and permeability.
The lack of soil structure reduces the availability of oxygen and oxygenation capacity in the root zone, limiting plant growth. The hard crust that is generated when these soils dry out hinders the germination process and prevents the normal growth of the roots.

Na ions inhibit the assimilation of K ions, which will be expressed in the crop as a higher K deficiency, lowering the enzymatic activity of the plant and protein synthesis.
On the other hand, the increase in pH affects the availability of certain essential nutrients. The difficulty of water passing through deflocculation or sodium humate crusts causes water stress in the plant.
Of course, in the soil, sodium can also be harmful; through the PSI we can determine if that Sodium could be a problem in the agricultural soils that we are managing.
Specifically, the affectations by sodium in the soil can be grouped as follows:
Effects on soil properties:
- Physical: The sodicity leads to the electrical double layer being expanded. The expansion of the double layer, due to the effects of valence and high hydrated radius of the Na+ ion, produces dispersion and there is no formation of aggregates. As no structure is generated (lack of porosity) the movement of water in this type of soil is very slow and the hydraulic conductivity is very low. On the surface, if there is the presence of moderately fine to fine textures, a laminar-type structure with strong crusting is generated, making it difficult for the crop to emerge.
- Chemicals: In the soil solution there is a high concentration of sodium salts capable of undergoing alkaline hydrolysis, of the sodium carbonate and bicarbonate type. These highly soluble salts produce increases in soil pH to values equal to or greater than 8.5 (strongly alkaline soils), which severely hinders the availability of most nutrients as well as biological activity. Sodic soils usually have “black crusts” on their surface, which are sodium humates produced by the union of colloidal organic matter with the sodium present. These black crusts are easily identified on the soil surface, allowing the problem of sodicity to be recognized with the naked eye.
 AgronoBlog – Agriculture Blog
AgronoBlog – Agriculture Blog 


