Electrical conductivity is the most extended and widely used parameter in salinity estimation. It is based on the speed with which the electric current passes through a salt solution, which is proportional to the concentration of salts in solution. Until a few years ago it was expressed in mmhos/cm. It is currently expressed in dS/m (dS=deciSiemens), both measurements being equivalent (1 mmhos/cm = 1 dS/m).
Electrical conductivity is defined as the ability of inorganic salts in solution (electrolytes) to conduct electrical current. Pure water practically does not conduct current, however, water with dissolved salts conducts electricity. The positively and negatively charged ions are the ones that conduct the current and the amount conducted will depend on the number of ions present and their mobility. In most aqueous solutions, the greater the amount of dissolved salts, the greater the conductivity, this effect continues until the solution is so full of ions that freedom of movement is restricted and the conductivity may decrease rather than increase. , giving cases of two different concentrations with the same conductivity.
Electrical conductivity of the soil
When speaking of electrical conductivity of the soil, reference is usually made to the electrical conductivity of its saturation extract. And electrical conductivity, as such, is determined in a liquid medium. It is assumed, although this has not yet been demonstrated, that said conductivity corresponds to the electrical conductivity of the interstitial fluid of the soil. This last statement is flawed. To determine the electrical conductivity of a soil, it is necessary to add more water and the latter contributes to dilute the salt content of the interstitial solution, lowering its original conductivity.
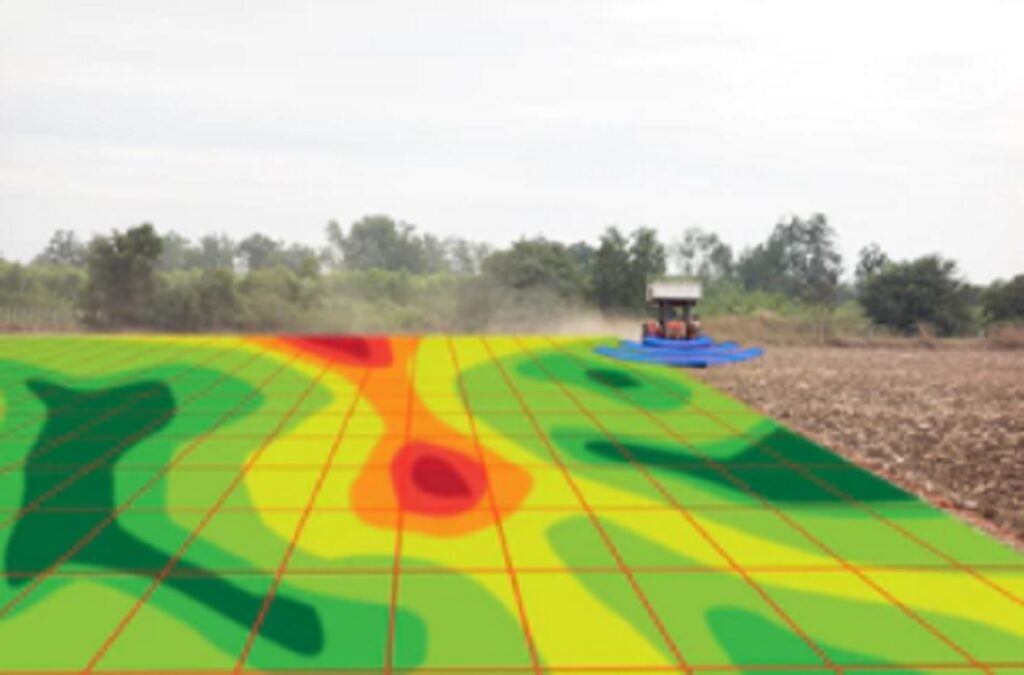
When a soil is new and begins to fertilize, the interior of the clods is always poorer in nutrients and therefore its conductivity is lower than that of the nutrient solution that is being applied. Over time, the soil becomes saturated with nutrients and the interior of the clods begins to be richer than the exterior. These differences mean that the interpretation of an extract from Saturation to Field Capacity must be carried out carefully taking these factors into account. Some substances ionize more completely than others and therefore conduct current better. Each acid, base or salt has its characteristic curve of concentration against conductivity. They are good conductors: acids, bases and inorganic salts: HC1, NaOH, NaC1, Na2CO3, etc. They are bad conductors: The molecules of organic substances that due to the nature of their bonds are non-ionic: such as sucrose, benzene, hydrocarbons, carbohydrates, etc., these substances do not ionize in water and therefore do not They conduct electrical current. An increase in temperature lowers the viscosity of water and allows the ions to move faster, conducting more electricity. This effect of temperature is different for each ion, but typically for dilute aqueous solutions, conductivity varies from 1 to 4% per °C. Knowing these factors, measuring conductivity allows us to get a very rough idea of how much of dissolved salts.
 AgronoBlog – Agriculture Blog
AgronoBlog – Agriculture Blog 


